VACCINE MANUFACTURING FACILITY DESIGN
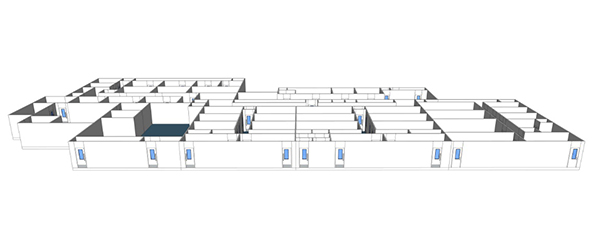
DESIGNING A VACCINE MANUFACTURING FACILITY
Like any other pharmaceutical or biotechnology manufacturing environment, vaccine production requires dedicated cleanroom areas for their vaccine manufacturing facility. As seen in our article designing a CGMP Cleanroom for Cell & Gene Therapy, manufacturing biologic drugs brings challenges to the cleanroom design. Vaccine manufacturing facility design and construction is no exception to this rule.
This article will cover many aspects to help you understand how to plan a vaccine facility layout. You will find pieces of information about:
General cleanroom considerations for a GMP Facility Design
Different types of vaccines
Vaccine manufacturing facility detailed layout
Types of equipment needed for vaccine production
The different steps for manufacturing a viral vector vaccine
And vaccine-related products manufactured in cleanrooms
All stages of vaccine development and manufacturing need cleanroom environments for delivering the required critical environment. Proper temperature, humidity, airflow, air pressure (positive or negative depending on the BSL level), and other environmental requirements are mandatory to provide a safe vaccine without any risk of contamination.
Here are a few things to take into consideration for a vaccine manufacturing facility design or biomanufacturing facility layout:
Type of vaccines or components you manufacture
Biosafety Levels (BSL) depending on the type of vaccines or viral vector used
Workflow patterns
ISO classifications / GMP requirements for vaccine cleanroom
Process stage (R&D, preclinical and clinical stages, large-scale manufacturing)
Dimensions, equipment inside the room, number of people working inside