Adoptive Cell Therapies
Immatics’ clinical ACTengine® programs are based on genetically engineering a patient’s own, autologous T cells with novel TCRs designed to recognize the cancer target on the tumor. The engineered T cells (TCR-T) aim to induce a robust and specific anti-tumor attack to fight the cancer.
ACTengine®at a glance
Immatics’ clinical product class ACTengine® is a personalized approach in which the patient’s own T cells (autologous) are genetically modified to express a novel proprietary TCR, an approach also known as TCR-T (T cell receptor-engineered T cell). The modified T cells are then reinfused into the patient to specifically engage with the tumor. The product class ACTallo® is advancing the ACT concept beyond individualized manufacturing and is being developed to generate allogeneic “off-the-shelf” cell therapies.
EXPANDED TARGET SPACE COMPARED TO CAR-T
ACTengine® TCR-T product candidates target tumor-associated peptides presented by HLA-molecules on the tumor cell surface. Most relevant solid cancer targets are of intracellular nature and can only be accessible by TCR-based approaches.
TCRS WITH DESIRABLE AFFINITY AND SPECIFICITY
ACTengine® TCRs identified via the XCEPTOR® TCR discovery platform and characterized via XPRESIDENT® guided on- and off-target toxicity screening platform show desirable affinity and high specificity for their cancer cell target. Immatics’ competitive advantage to other TCR-T approaches is our ability to combine the most suitable target (via XPRESIDENT®) with the right TCR (via XCEPTOR®).
ACTIVE AT PHYSIOLOGICAL LEVELS OF TARGET EXPRESSION
Immatics believes that ACTengine® TCR-T product candidates are highly potent and capable of inducing the killing of tumor cells that present the target at physiological target copy numbers identified by quantitative mass spectrometry and TCR validation.
OPTIMIZED MANUFACTURING
Immatics’ proprietary short manufacturing process, which includes a significantly shorter manufacturing period and a proprietary cytokine cocktail used to promote T cell expansion in cell culture, is designed to produce younger, less differentiated T cell phenotypes that are associated with better T cell engraftment and persistence at the site of the tumor.
CLINICAL TRIALS
The objective of the two Phase 1 clinical trials with the ACTengine® product candidates IMA201 and IMA203 is to evaluate safety, tolerability and initial signs of clinical and biological efficacy in target-positive solid cancer patients and to determine a recommended Phase 2 dose. The two clinical trials continue to actively recruit patients.
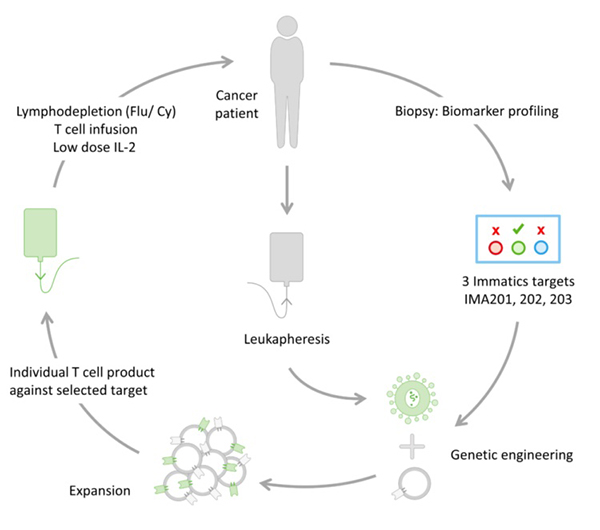
There are several reasons why a patient’s own T cells are often not able to protect the body against cancer. These include the unavailability of activated tumor antigen specific T cells or the insufficient affinity of endogenous target-specific TCRs to properly activate the T cell and destroy the tumor. Immatics’ approach to solving these problems is to engineer autologous T cells with a well characterized and potent TCR, which is the underlying principle of its ACTengine® program.
ACTengine® is based on genetically engineering a patient’s own T cells with a novel TCR designed to recognize the cancer target identified by Immatics’ XPRESIDENT® platform. If the target of interest is confirmed on a patient’s tumor by the IMADetect® companion diagnostic device candidate, lentiviral transduction of the patient’s autologous T cells with a target specific exogenous TCR essentially “reprograms” the T cells to attack the tumor. The engineered T cells are then multiplied in vitro and reinfused back into the patient to treat the tumor.
To activate T cell proliferation and to facilitate T cell engraftment and persistence, Immatics’ current ACTengine® clinical trials include a preconditioning process called lymphodepletion before the engineered T cells are infused into the body. Following infusion of the ACTengine® product candidate, a low dose of IL-2, an immune system signaling molecule, is administered for 14 days to further enhance persistence of the infused engineered T cells.