Pharmaceutical Packaging Design and Optimization
Pharmaceutical Packaging Design and Optimization
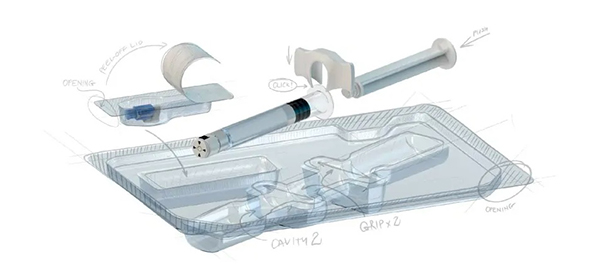
It is essential that medical device and pharmaceutical secondary packaging creates a sterile barrier from environmental hazards and allows for efficient and effective sterilization without the risk of degrading the packaging material. The stability of the material throughout the supply chain must be ensured as well as the safe and secure transportation of the product to avoid any damage during transportation which could result in a loss of seal integrity. Packaging can also serve other functions such as protecting against moisture and light, providing easy or specifically designed extraction of the product from the packaging, directing the user to a specific sequence of using a multicomponent set, marking, identification and increased ergonomics for medical personnel. The requirements for the packaging of medical devices is precisely specified by the US FDA (FDA 21 CFR, Part 820) and Medical Device Directive for EU (MDD Council Directive 93/42/EEC MDD 93/42/EEC, Annex II. EN-ISO 11607) standard.
Whilst focusing on core activities like device or Active Pharmaceutical Ingredient (API) manufacturing, many companies decide to outsource packaging design in order to receive the best support and reduced lead-time for this non-core part of their business. Packaging systems should be designed to minimize the safety hazard to the end user and patient whilst under the intended conditions of use. To accomplish this, we apply risk analysis techniques throughout the entire design and development system right through to commercialization to ensure our solutions are reliable and stable.
Medical devices and pharmaceutical products along with their packaging, may vary from micro-sizes to large, multi-item sets. SteriPack offers fully integrated single-source solutions starting with concept design as well as design development, expertise and assistance to meet the needs and requirements of the product, customer and notified bodies. Material selection is crucial when designing for your sterile barrier system and your specific requirements for product safety and stability in time. Our packaging design experts can help you identify the appropriate material for each unique customer application as well as cost effective and high-quality solutions.
Our knowledge and expertise lies in a range of areas:
- Packaging concept design (molded, thermoformed plastic and cardboard packaging)
- Packaging optimization (size and functionality)
- Concept assessment vs input / technology / cost
- Functional packaging prototype manufacturing (3D printed prototypes, plotter cardboard boxes, etc.)
- Proof of concept
- Packaging material selection
- Packaging design development and design review
- 3D modelling
- 2D drawings
- Packaging prototypes for trail fabrication created using prototype tools
We offer:
- Single and double sterile barrier packaging design and development for medical devices
- Secondary packaging design and development for pharmaceutical products
Shelf cartons, cases and shipper boxes sourced by SteriPack from local suppliers come with FSC (Forest Steward Council) certificate.