Topical Formulation Development
Topical Formulation Development
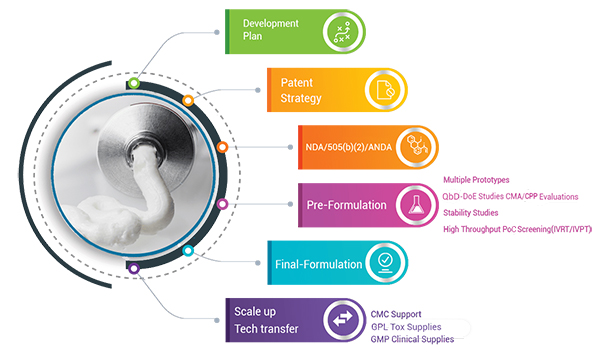
Tergus offers comprehensive solutions for your topical formulation development needs. We have expertise working with a range of Active Pharmaceutical Ingredient (APIs) including all BCE class compounds (especially poorly soluble and poorly permeable compounds), New Chemical Entities (NCEs), small and large molecules, pro-drugs, proteins, peptides, enzymes, Spherical Nucleic Acid (SNA), microbiomes, and high potency compounds. Tergus provides reproducible and scalable formulations by using quality-by-design and risk mitigation to produce a robust topical dosage form appropriate for proof-of-concept, GLP, clinical trials (Phase I, II, III), and subsequent commercial manufacturing. We can ensure the fastest path to market by customizing each development path to meet your phase-appropriate needs, allowing us to reach critical “Go/No Go” decisions earlier and achieve faster results.
Our Topical Formulation Services Include
Tergus is a one-stop solution for your topical formulation development needs. Scientists at Tergus come from a variety of backgrounds including topical/transdermal skin delivery, pharmacology, topical formulation development, biology and pharmaceutical sciences.
Formulation Development
- Complete NCE Generic and OTC product development
- Repurposing Phase I or II dosage form clinical setbacks via turnkey formulation solutions
- Manufacturing process optimization using QbD and CFR-compliant statistical software
- Evaluation of innovative platforms and technologies
- Expertise in developing non-infringing novel patentable formulations
- IP review and risk mitigation strategies
- Pre-clinical toxicology support
- Addressing FDA deficiencies
- Container closure selections
- 510(k) medical devices