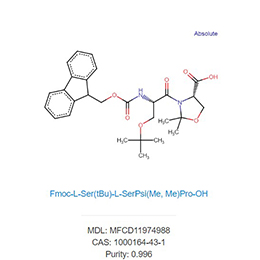
Pharmaceutical Formulations
Pharmaceutical drug formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
Pharmaceutical Formulation development is a key area of product development that can determine patentability, lifecycle and, ultimately, the success of a pharmaceutical product. Companies integrate formulation development functions and personnel into their product development cycle in many different ways. In fully integrated large pharmaceutical companies, specific departments may exist to deal with such areas as the physical characterization of drug substances and formulation issues. In many cases, other departments, often in other buildings or locations, may handle preclinical functions, such as animal testing. These departments must work closely to gain a complete understanding of their unique drug substance. For smaller, emerging companies, as well as many biotechnology companies, the function of formulation development often falls in one of several departments. Sometimes it exists within project group responsible for the overall development plan of a particular drug candidate. These groups are led by project managers who interface with both internal and external resources. The analytical chemistry or the analytical development lab often executes the majority of the formulation tasks, as they are already the ‘experts’ in the analytical assays, most often high performance liquid chromatography (HPLC) assays, which are an integral part of product development.
Below is a list of suppliers who specialise in providing Pharmaceutical Formulations:
Pharmaceutical Formulations Products
Search by Country
China
Egypt
India
United States
Germany
Ukraine
Switzerland
Austria
United Kingdom
Nigeria
Zimbabwe
Tunisia
Morocco
South Africa
Romania
Guatemala
Indonesia
Czech Republic
Canada
Angola
Argentina
Albania
Bulgaria
Ireland
Namibia
Latvia
Italy
Uganda
Uruguay
Pakistan
Turkey
Algeria
Belgium
Singapore
Australia
Jordan
Denmark
Bangladesh
Hungary
Greece
Sweden
Peru
Thailand
Ghana
New Zealand
Poland
Japan
Spain
Saudi Arabia
Netherlands
Search by Supplier
 Jurabek Laboratories Ltd
(5)
Jurabek Laboratories Ltd
(5)
 WuXi STA
(4)
WuXi STA
(4)
 Pharco Pharmaceuticals
(4)
Pharco Pharmaceuticals
(4)
 Kusum Healthcare Pvt Ltd
(5)
Kusum Healthcare Pvt Ltd
(5)
 Jamnadas Industries
(4)
Jamnadas Industries
(4)
 Flagship
(5)
Flagship
(5)
 Amerilab Technologies
(2)
Amerilab Technologies
(2)
 ZwickRoell Pvt. Ltd
(5)
ZwickRoell Pvt. Ltd
(5)
 Steierl-Pharma GmbH
(4)
Steierl-Pharma GmbH
(4)
 Materials Lab
(5)
Materials Lab
(5)
 Chaitanya Pharmaceuticals Pvt. Ltd.
(3)
Chaitanya Pharmaceuticals Pvt. Ltd.
(3)
 Camber Pharmaceuticals, Inc
(4)
Camber Pharmaceuticals, Inc
(4)
 AlpVision
(10)
AlpVision
(10)
 Genericon Pharma
(5)
Genericon Pharma
(5)
 Contipro a.s.
(5)
Contipro a.s.
(5)
 Velesco Pharmaceutical Services
(4)
Velesco Pharmaceutical Services
(4)
 Vectura Group Ltd
(7)
Vectura Group Ltd
(7)
 Shalina Healthcare
(5)
Shalina Healthcare
(5)
 Osworth Nigeria Limited
(5)
Osworth Nigeria Limited
(5)
 Ipca Laboratories
(2)
Ipca Laboratories
(2)
 Babuline Pharma Pvt. Ltd
(5)
Babuline Pharma Pvt. Ltd
(5)
 Fidson Healthcare Plc
(2)
Fidson Healthcare Plc
(2)
 Zimbabwe Pharmaceuticals (PVT) LTD
(5)
Zimbabwe Pharmaceuticals (PVT) LTD
(5)
 TERIAK
(7)
TERIAK
(7)
 Steripharma
(5)
Steripharma
(5)
 MAXNOVA HEALTHCARE
(5)
MAXNOVA HEALTHCARE
(5)
 FARMAPRIM
(5)
FARMAPRIM
(5)
 Pfanstiehl
(4)
Pfanstiehl
(4)
 Nanz Med Science Pharma (P) Ltd
(3)
Nanz Med Science Pharma (P) Ltd
(3)
 Datlabs (Pvt) Ltd
(4)
Datlabs (Pvt) Ltd
(4)
 Nexus Pharma Co., Ltd.
(5)
Nexus Pharma Co., Ltd.
(5)
 MJ Labs
(4)
MJ Labs
(4)
 Kivonyx Healthcare
(5)
Kivonyx Healthcare
(5)
 Biotehnos
(5)
Biotehnos
(5)
 BIOFARM
(5)
BIOFARM
(5)
 Albia Biocare
(5)
Albia Biocare
(5)
 PCL Guatemala
(5)
PCL Guatemala
(5)
 Dexa Group
(5)
Dexa Group
(5)
 Adcock Ingram
(5)
Adcock Ingram
(5)
 Fagron a.s.
(4)
Fagron a.s.
(4)
 Heaton
(9)
Heaton
(9)
 Quagen Pharmaceuticals
(5)
Quagen Pharmaceuticals
(5)
 Stemcell Technologies
(5)
Stemcell Technologies
(5)
 Prince Farma, Lda
(8)
Prince Farma, Lda
(8)
 Laboratorios Bernabó
(4)
Laboratorios Bernabó
(4)
 Laboratorio Varifarma SA
(4)
Laboratorio Varifarma SA
(4)
 Laboratorio Elea
(5)
Laboratorio Elea
(5)
 Denver Farma Sa
(3)
Denver Farma Sa
(3)
 Secant Group, LLC
(5)
Secant Group, LLC
(5)
 Lekli Pharma
(7)
Lekli Pharma
(7)
 CPS Pharma GmbH
(4)
CPS Pharma GmbH
(4)
 Brunel Laboratories (Pty) Ltd
(5)
Brunel Laboratories (Pty) Ltd
(5)
 Amring Pharmaceuticals Inc
(2)
Amring Pharmaceuticals Inc
(2)
 BB-NCIPD Ltd
(4)
BB-NCIPD Ltd
(4)
 Perrigo Company
(5)
Perrigo Company
(5)
 Fabupharm (Pty) Ltd.
(4)
Fabupharm (Pty) Ltd.
(4)
 Medfau sh.p.k
(5)
Medfau sh.p.k
(5)
 Lotos Pharma SIA
(5)
Lotos Pharma SIA
(5)
 Arwan Pharmaceutical Industries Lebanon s.a.l.
(5)
Arwan Pharmaceutical Industries Lebanon s.a.l.
(5)
 Agary Pharmaceuticals Ltd
(5)
Agary Pharmaceuticals Ltd
(5)
 Benta Pharma Industries - BPI
(5)
Benta Pharma Industries - BPI
(5)
 Bayer Vital GmbH
(5)
Bayer Vital GmbH
(5)
 Farmigea SpA
(9)
Farmigea SpA
(9)
 Lekhim
(5)
Lekhim
(5)
 Erongomed
(3)
Erongomed
(3)
 Quotient Sciences
(9)
Quotient Sciences
(9)
 Globela Pharma Pvt Ltd
(8)
Globela Pharma Pvt Ltd
(8)
 Rene Industries Ltd
(5)
Rene Industries Ltd
(5)
 Laboratory ION SA
(5)
Laboratory ION SA
(5)
 Bio Labs Pvt Ltd.
(5)
Bio Labs Pvt Ltd.
(5)
 Biem Ilac
(5)
Biem Ilac
(5)
 Kilitch Drugs (India) Ltd
(4)
Kilitch Drugs (India) Ltd
(4)
 CAPS (Private) Limited
(5)
CAPS (Private) Limited
(5)
 Zodel Biotec Ltd
(4)
Zodel Biotec Ltd
(4)
 Laboratorio Libra S.A.
(5)
Laboratorio Libra S.A.
(5)
 Novamedika
(3)
Novamedika
(3)
 Typharm Group
(5)
Typharm Group
(5)
 Ooo Niarmedik Farma
(1)
Ooo Niarmedik Farma
(1)
 Genericlab Sarl
(4)
Genericlab Sarl
(4)
 Engelhard Arzneimittel GmbH & Co. KG
(4)
Engelhard Arzneimittel GmbH & Co. KG
(4)
 Helix Pharma (Pvt) Limited
(5)
Helix Pharma (Pvt) Limited
(5)
 GENIX PHARMA PRIVATE LIMITED
(4)
GENIX PHARMA PRIVATE LIMITED
(4)
 Cirondrugs
(2)
Cirondrugs
(2)
 Nutrisan
(5)
Nutrisan
(5)
 El Kendi Pharmaceutical
(5)
El Kendi Pharmaceutical
(5)
 CAMFIL
(5)
CAMFIL
(5)
 STADA Pharmaceuticals Australia
(10)
STADA Pharmaceuticals Australia
(10)
 Axantia
(9)
Axantia
(9)
 FINAR
(8)
FINAR
(8)
 Hanmi Pharmaceutical
(4)
Hanmi Pharmaceutical
(4)
 JGL pharmaceutical company
(5)
JGL pharmaceutical company
(5)
 APIPHARMA Ltd.
(5)
APIPHARMA Ltd.
(5)
 Macure Pharma
(4)
Macure Pharma
(4)
 Healthcare Pharmaceuticals
(5)
Healthcare Pharmaceuticals
(5)
 Gedeon Richter Plc
(4)
Gedeon Richter Plc
(4)
 Eriochem SA
(4)
Eriochem SA
(4)
 Elpen
(5)
Elpen
(5)
 CSPC Pharmaceutical Group Limited
(4)
CSPC Pharmaceutical Group Limited
(4)
 Alfa Laval Corporate AB
(5)
Alfa Laval Corporate AB
(5)
 Tulipanesa Peru S.A.C.
(5)
Tulipanesa Peru S.A.C.
(5)
 Taj Pharma India Ltd, Sarigam
(1)
Taj Pharma India Ltd, Sarigam
(1)
 Paradigm Pharmaceuticals Inc
(5)
Paradigm Pharmaceuticals Inc
(5)
 Sparsha Pharma International Pvt. Ltd.
(5)
Sparsha Pharma International Pvt. Ltd.
(5)
 LABORATORI BALDACCI SPA
(4)
LABORATORI BALDACCI SPA
(4)
 Farmaceutici Damor Spa
(5)
Farmaceutici Damor Spa
(5)
 Ocular Therapeutix, Inc.
(9)
Ocular Therapeutix, Inc.
(9)
 Evonik Industries
(2)
Evonik Industries
(2)
 Valeo Pharma Inc
(4)
Valeo Pharma Inc
(4)
 VIOSER S.A.
(4)
VIOSER S.A.
(4)
 PROFARMA Sh.a
(5)
PROFARMA Sh.a
(5)
 CFO Pharma
(5)
CFO Pharma
(5)
 ALKALOID AD SKOPJE
(5)
ALKALOID AD SKOPJE
(5)
 GPSC Guilin Pharma Gh Ltd
(5)
GPSC Guilin Pharma Gh Ltd
(5)
 Celltrion Healthcare
(5)
Celltrion Healthcare
(5)
 Beximco Pharmaceuticals Ltd
(5)
Beximco Pharmaceuticals Ltd
(5)
 Amponsah-Efah Pharmaceutical Company
(5)
Amponsah-Efah Pharmaceutical Company
(5)
 AGP Limited
(5)
AGP Limited
(5)
 AFT Pharmaceuticals
(5)
AFT Pharmaceuticals
(5)
 Aflofarm
(5)
Aflofarm
(5)
 Beacon Converters
(10)
Beacon Converters
(10)
 Ego Pharmaceuticals
(5)
Ego Pharmaceuticals
(5)
 Meiji Holdings Co., Ltd.
(4)
Meiji Holdings Co., Ltd.
(4)
 VeKo Care
(5)
VeKo Care
(5)
 Siegfried AG
(5)
Siegfried AG
(5)
 Norbrook® Laboratories Limited
(5)
Norbrook® Laboratories Limited
(5)
 Healthy Life Pharma Private Limited.
(5)
Healthy Life Pharma Private Limited.
(5)
 COMASA
(5)
COMASA
(5)
 MSN Laboratories Private Limited
(1)
MSN Laboratories Private Limited
(1)
 Tapemark
(7)
Tapemark
(7)
 Doppel Pharma
(2)
Doppel Pharma
(2)
 Nectar Lifesciences Ltd
(2)
Nectar Lifesciences Ltd
(2)
 Orchid Pharma Ltd.
(2)
Orchid Pharma Ltd.
(2)
 Octapharma Biopharmaceuticals GmbH
(5)
Octapharma Biopharmaceuticals GmbH
(5)
 Seda Pharmaceutical Development Service
(5)
Seda Pharmaceutical Development Service
(5)
 Nanopharm Limited.
(5)
Nanopharm Limited.
(5)
 Bradford
(1)
Bradford
(1)
 Sfm medical devices GmbH
(5)
Sfm medical devices GmbH
(5)
 Optimus Pharma
(2)
Optimus Pharma
(2)
 Jamjoom Pharmaceuticals Co.
(5)
Jamjoom Pharmaceuticals Co.
(5)
 Gland Pharma Limited
(2)
Gland Pharma Limited
(2)
 Akorn Inc
(4)
Akorn Inc
(4)
 IMCD Pharmaceuticals
(5)
IMCD Pharmaceuticals
(5)
 Hovione Holding AG
(5)
Hovione Holding AG
(5)
 EffRx Pharmaceuticals SA
(3)
EffRx Pharmaceuticals SA
(3)
 DIPHARMA SA
(4)
DIPHARMA SA
(4)
 Exelead
(5)
Exelead
(5)
 QProducts & Services
(2)
QProducts & Services
(2)
 Coriolis Pharma
(6)
Coriolis Pharma
(6)
 JPT Peptide Technologies
(5)
JPT Peptide Technologies
(5)
 Nova Laboratories
(3)
Nova Laboratories
(3)
 AZZUR GROUP
(2)
AZZUR GROUP
(2)
 H&T Presspart Manufacturing Ltd
(3)
H&T Presspart Manufacturing Ltd
(3)
 Dermal Laboratories Ltd
(5)
Dermal Laboratories Ltd
(5)
 Air Products Inc.
(2)
Air Products Inc.
(2)
 Neon Laboratories Ltd
(5)
Neon Laboratories Ltd
(5)
 Medopharm Pvt Ltd
(4)
Medopharm Pvt Ltd
(4)
 Azurity Pharmaceuticals, Inc
(5)
Azurity Pharmaceuticals, Inc
(5)
 Anantco Enterprises Pvt Ltd
(5)
Anantco Enterprises Pvt Ltd
(5)
 Tillotts Pharma AG
(4)
Tillotts Pharma AG
(4)
 Korean Drug Co
(5)
Korean Drug Co
(5)
 Gelpell AG
(4)
Gelpell AG
(4)
 Croma-Pharma
(5)
Croma-Pharma
(5)
 Sinergium Biotech
(2)
Sinergium Biotech
(2)
 PT. Meiji Indonesian Pharmaceutical Industries
(5)
PT. Meiji Indonesian Pharmaceutical Industries
(5)
 PT. AstraZeneca Indonesia
(4)
PT. AstraZeneca Indonesia
(4)
 Promea Therapeutics
(5)
Promea Therapeutics
(5)
 Labotec (Pty) Ltd - Midrand Head Office
(5)
Labotec (Pty) Ltd - Midrand Head Office
(5)
 Otsuka Pharmaceutical Co., Ltd.
(5)
Otsuka Pharmaceutical Co., Ltd.
(5)
 Swiss Pharma Nigeria Ltd
(3)
Swiss Pharma Nigeria Ltd
(3)
 Rubicon Research Pvt. Ltd
(4)
Rubicon Research Pvt. Ltd
(4)
 Pharma Q (Pty) Ltd
(3)
Pharma Q (Pty) Ltd
(3)
 Rhodes Pharmaceuticals
(5)
Rhodes Pharmaceuticals
(5)
 Astron Limited
(4)
Astron Limited
(4)
 Catalent, Inc
(2)
Catalent, Inc
(2)
 MVIC
(5)
MVIC
(5)
 VGXI, Inc
(3)
VGXI, Inc
(3)
 Quay Pharma
(4)
Quay Pharma
(4)
 Avivia BV.
(3)
Avivia BV.
(3)
 Lonza
(5)
Lonza
(5)
 Adare Pharma Solutions
(5)
Adare Pharma Solutions
(5)
 Attwill Medical Solutions
(5)
Attwill Medical Solutions
(5)
 CordenPharma
(4)
CordenPharma
(4)
 Metrics Contract Services
(2)
Metrics Contract Services
(2)
 Altasciences
(4)
Altasciences
(4)
 Alcami Corporation, Inc.
(2)
Alcami Corporation, Inc.
(2)
 Recipharm
(5)
Recipharm
(5)
 CoreRx, Inc
(5)
CoreRx, Inc
(5)
 Bora Pharmaceuticals
(4)
Bora Pharmaceuticals
(4)
 Mikart LLC
(5)
Mikart LLC
(5)
 Selvok Pharmaceutical Co.
(5)
Selvok Pharmaceutical Co.
(5)
 Lubrizol Life Science
(5)
Lubrizol Life Science
(5)
 ProChem Inc
(5)
ProChem Inc
(5)